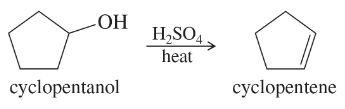
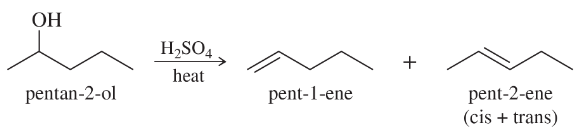
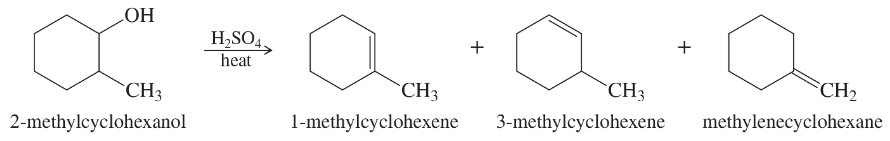
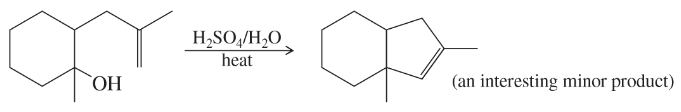
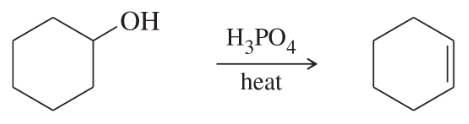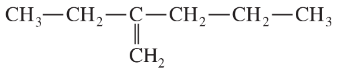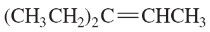Back
BackProblem 33g
Predict the products and mechanisms of the following reactions. When more than one product or mechanism is possible, explain which are most likely.
g. 1−bromo−1−methylcyclopentane + NaOEt in ethanol
Problem 33h
Predict the products and mechanisms of the following reactions. When more than one product or mechanism is possible, explain which are most likely.
h. 1-bromo-1-methylcyclopentane heated in methanol
Problem 34a
Propose mechanisms for the following reactions.
(a)
Problem 34b
Propose mechanisms for the following reactions.
(b)
Problem 34c
Propose mechanisms for the following reactions.
(c)
Problem 34d
Propose mechanisms for the following reactions.
(d)
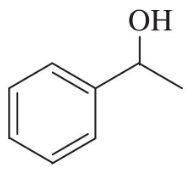
Problem 35a
Show the product(s) you expect from dehydration of the following alcohols when they are heated in sulfuric or phosphoric acid. In each case, use a mechanism to show how the products are formed.
(a)
Problem 35b
Show the product(s) you expect from dehydration of the following alcohols when they are heated in sulfuric or phosphoric acid. In each case, use a mechanism to show how the products are formed.
(b)
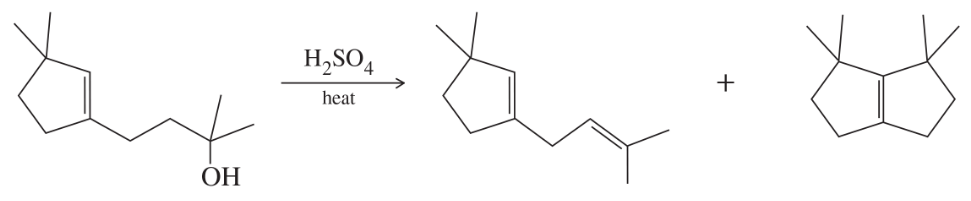
Problem 35c
Show the product(s) you expect from dehydration of the following alcohols when they are heated in sulfuric or phosphoric acid. In each case, use a mechanism to show how the products are formed.
(c)
Problem 36a
The dehydrogenation of butane to trans-but-2-ene has ΔH° = +116 kJ/mol (+27.6 kcal/mol) and ΔS° = +117J/kelvin-mol (+28.0 cal/kelvin-mol).
a. Compute the value of ΔG° for dehydrogenation at room temperature (25 °C or 298 °K). Is dehydrogenation favored or disfavored?
HINT: When you are doing synthesis problems, avoid using these high-temperature industrial methods. They require specialized equipment, and they produce variable mixtures of products.
Problem 37a
For practice in recognizing mechanisms, classify each reaction according to the type of mechanism you expect:
1. Free-radical chain reaction
2. Reaction involving strong bases and strong nucleophiles
3. Reaction involving strong acids and strong electrophiles
(a)
Problem 37b
For practice in recognizing mechanisms, classify each reaction according to the type of mechanism you expect:
1. Free-radical chain reaction
2. Reaction involving strong bases and strong nucleophiles
3. Reaction involving strong acids and strong electrophiles
(b)
Problem 37c
For practice in recognizing mechanisms, classify each reaction according to the type of mechanism you expect:
1. Free-radical chain reaction
2. Reaction involving strong bases and strong nucleophiles
3. Reaction involving strong acids and strong electrophiles
(c)
Problem 37d
For practice in recognizing mechanisms, classify each reaction according to the type of mechanism you expect:
1. Free-radical chain reaction
2. Reaction involving strong bases and strong nucleophiles
3. Reaction involving strong acids and strong electrophiles.
(d)
Problem 38a
Propose mechanisms for the following reactions. Additional products may be formed, but your mechanism only needs to explain the products shown.
(a)
(Hint: Hydride shift)
Problem 38c
Propose mechanisms for the following reactions. Additional products may be formed, but your mechanism only needs to explain the products shown.
(c)
Problem 39a
Propose mechanisms for the following reactions.
(a)
HINT: Alcohol dehydrations usually go through E1 elimination of the protonated alcohol, with a carbocation intermediate. Rearrangements are common.
Problem 39b
Propose mechanisms for the following reactions.
(b)
HINT: Alcohol dehydrations usually go through E1 elimination of the protonated alcohol, with a carbocation intermediate. Rearrangements are common.
Problem 39d
Propose mechanisms for the following reactions.
d.
HINT: Alcohol dehydrations usually go through E1 elimination of the protonated alcohol, with a carbocation intermediate. Rearrangements are common.
Problem 40a,b,c
Draw a structure for each compound (includes old and new names).
a. 3-methylpent-1-ene
b. cis-3-methyl-3-hexene
c. 3,4-dibromobut-1-ene
Problem 40d,e,f
Draw a structure for each compound (includes old and new names).
d. 1,3-cyclohexadiene
e. cycloocta-1,4-diene
f. (Z)-3-methyl-2-octene
Problem 40g,h
Draw a structure for each compound (includes old and new names).
g. vinylcyclopropane
h. (Z)-2-bromo-2-pentene
Problem 40i
Draw a structure for each compound (includes old and new names).
i. (3Z,6E)-1,3,6-octatriene
Problem 41
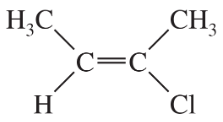
Label each structure as Z, E, or neither.
(a)
(b)
(c)
(d)
Problem 42a,b
Determine which compounds show cis-trans isomerism. Draw and label the isomers, using both the cis-trans and E-Z nomenclatures where applicable.
a. pent-1-ene
b. pent-2-ene
Problem 42c,d
Determine which compounds show cis-trans isomerism. Draw and label the isomers, using both the cis-trans and E-Z nomenclatures where applicable.
c. hex-3-ene
d. 1,1-dibromopropene
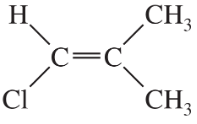
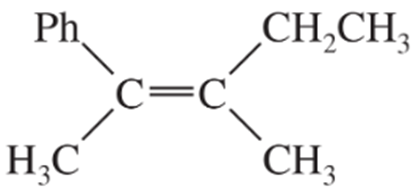
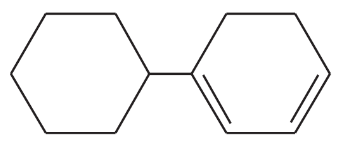
Problem 43a,b
Give a correct name for each compound.
(a)
(b)
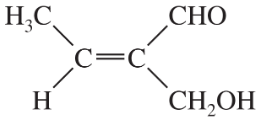
Problem 43c,d
Give a correct name for each compound.
(c)
(d)
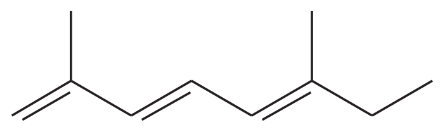
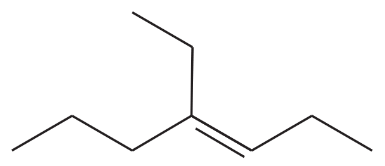
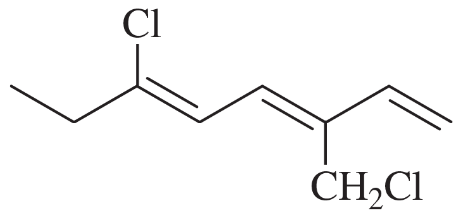
Problem 43e,f
Give a correct name for each compound.
(e)
(f)
Problem 44a
Draw and name all five isomers of formula C3H5F.