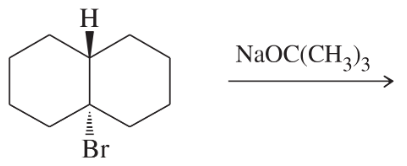
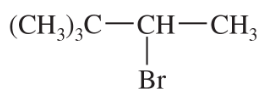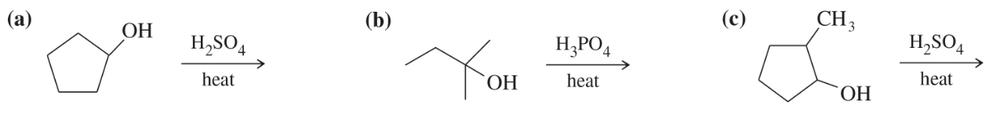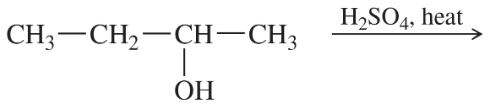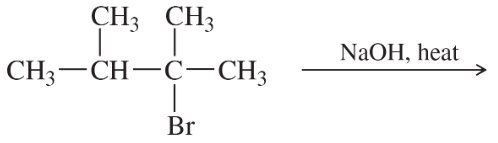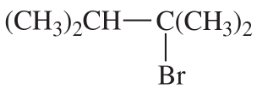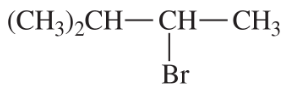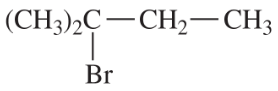Back
BackProblem 44b
Draw all 12 acyclic (no rings) isomers of formula C4H7Br. Include stereoisomers.
Problem 44c
Cholesterol, C27H46O, has only one pi bond. With no additional information, what else can you say about its structure?
Problem 45a
Draw and name all stereoisomers of 3-chlorohepta-2,4-diene
a. using the cis-trans nomenclature.
Problem 45b
Draw and name all stereoisomers of 3-chlorohepta-2,4-diene
b. using the E-Z nomenclature.
Problem 46a
For each alkene, indicate the direction of the dipole moment. For each pair, determine which compound has the larger dipole moment.
a. cis-1,2-difluoroethene or trans-1,2-difluoroethene
Problem 46b
For each alkene, indicate the direction of the dipole moment. For each pair, determine which compound has the larger dipole moment
b. cis-1,2-dibromoethene or trans-2,3-dibromobut-2-ene
Problem 46c
For each alkene, indicate the direction of the dipole moment. For each pair, determine which compound has the larger dipole moment.
c. cis-1,2-dibromo-1,2-dichloroethene or cis-1,2-dichloroethene
Problem 47
The energy difference between cis- and trans-but-2-ene is about 4 kJ/mol; however, the trans isomer of 4,4-dimethylpent-2-ene is nearly 16 kJ/mol more stable than the cis isomer. Explain this large difference.
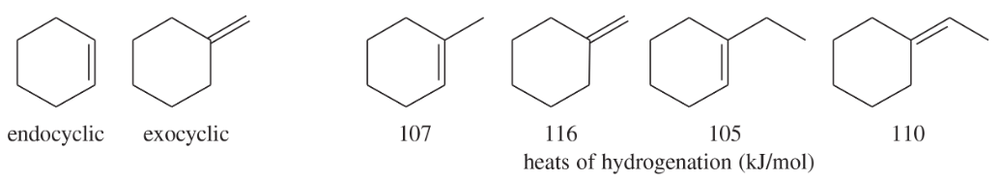
Problem 48
A double bond in a six-membered ring is usually more stable in an endocyclic position than in an exocyclic position. Hydrogenation data on two pairs of compounds follow. One pair suggests that the energy difference between endocyclic and exocyclic double bonds is about 9 kJ/mol. The other pair suggests an energy difference of about 5 kJ/mol. Which number do you trust as being more representative of the actual energy difference? Explain your answer.
Problem 49a
Predict the products of E1 elimination of the following compounds. Label the major products.
(a)
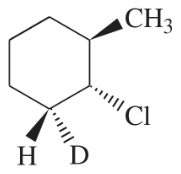
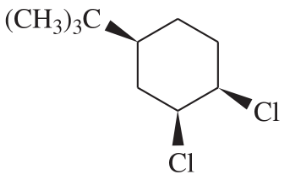
Problem 49c
Predict the products of E1 elimination of the following compounds. Label the major products.
(c)
Problem 50a,b,c
Predict the products formed by sodium hydroxide-promoted dehydrohalogenation of the following compounds. In each case, predict which will be the major product.
a. 1-bromobutane
b. 2-chlorobutane
c. 3-bromopentane
Problem 50d
Predict the products formed by sodium hydroxide-promoted dehydrohalogenation of the following compounds. In each case, predict which will be the major product.
d. cis-1-bromo-2-methylcyclohexane
Problem 50e
Predict the products formed by sodium hydroxide-promoted dehydrohalogenation of the following compounds. In each case, predict which will be the major product.
e. trans-1-bromo-2-methylcyclohexane
Problem 51a,b,c
What halides would undergo E2 dehydrohalogenation to give the following pure alkenes?
a. hex-1-ene
b. isobutylene
c. pent-2-ene
Problem 51d
What halides would undergo E2 dehydrohalogenation to give the following pure alkenes?
d. methylenecyclohexane
Problem 51e
What halides would undergo E2 dehydrohalogenation to give the following pure alkenes?
e. 4-methylcyclohexene
Problem 52a,b
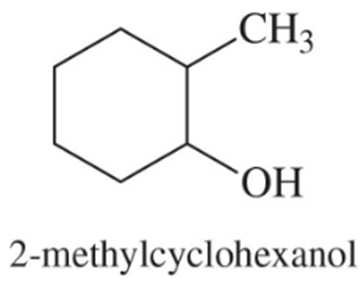
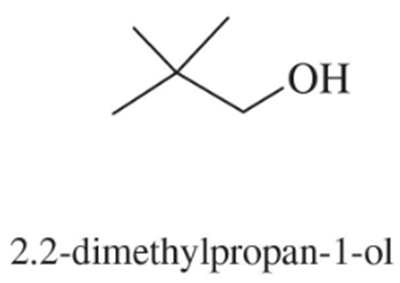
Predict the major products of acid-catalyzed dehydration of the following alcohols.
(a)
(b)
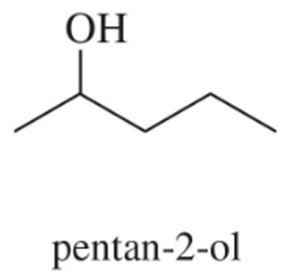
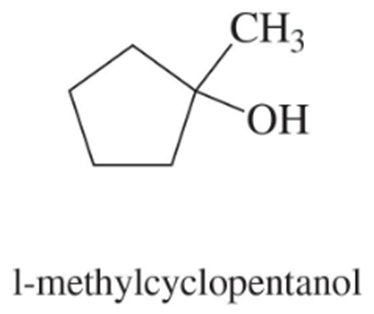
Problem 52c,d
Predict the major products of acid-catalyzed dehydration of the following alcohols.
(c)
(d)
Problem 53
Predict the products of the following reactions. When more than one product is expected, predict which will be the major product.
Problem 54a
Write a balanced equation for each reaction, showing the major product you expect.
(a)
Problem 54b
Write a balanced equation for each reaction, showing the major product you expect.
(b)
Problem 54c
Write a balanced equation for each reaction, showing the major product you expect.
(c)
Problem 54d
Write a balanced equation for each reaction, showing the major product you expect.
(d)
Problem 55a,b,c
Predict the dehydrohalogenation product(s) that result when the following alkyl halides are heated in alcoholic KOH. When more than one product is formed, predict the major and minor products.
(a)
(b)
(c)
Problem 55d,e
Predict the dehydrohalogenation product(s) that result when the following alkyl halides are heated in alcoholic KOH. When more than one product is formed, predict the major and minor products.
(d)
(e)
Problem 55f
Predict the dehydrohalogenation product(s) that result when the following alkyl halides are heated in alcoholic KOH. When more than one product is formed, predict the major and minor products.
(f)
Problem 56a,b,c
Using cyclohexane as your starting material, show how you would synthesize each of the following compounds. (Once you have shown how to synthesize a compound, you may use it as the starting material in any later parts of this problem.)
a. bromocyclohexane
b. cyclohexene
c. ethoxycyclohexane
Problem 56d,e,f
Using cyclohexane as your starting material, show how you would synthesize each of the following compounds. (Once you have shown how to synthesize a compound, you may use it as the starting material in any later parts of this problem.)
d. 3-bromocyclohex-1-ene
e. cyclohexa-1,3-diene
f. cyclohexanol
Problem 57a,b
Show how you would prepare cyclopentene from each compound.
a. cyclopentanol
b. cyclopentyl bromide