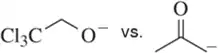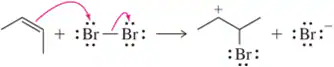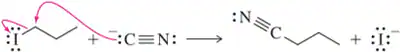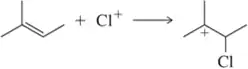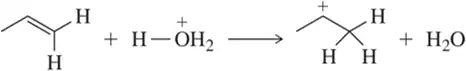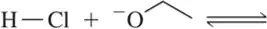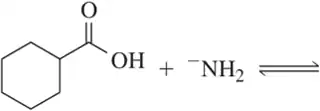Back
BackProblem 51a
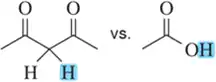
Identify the most stable conjugate base in each pair. Tell which structural features you analyzed and why you weighted them as you did in picking one answer.
(a)
Problem 51c
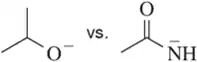
Identify the most stable conjugate base in each pair. Tell which structural features you analyzed and why you weighted them as you did in picking one answer.
(c)
Problem 51e
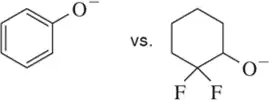
Identify the most stable conjugate base in each pair. Tell which structural features you analyzed and why you weighted them as you did in picking one answer.
(e)
Problem 51f
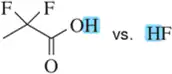
Identify the most stable conjugate base in each pair. Tell which structural features you analyzed and why you weighted them as you did in picking one answer.
(f)
Problem 52a
Identify the most acidic proton in each pair. Tell which structural features you analyzed and why you weighted them as you did in picking one answer. [Always start by drawing the conjugate base.]
(a)
Problem 52b
Identify the most acidic proton in each pair. Tell which structural features you analyzed and why you weighted them as you did in picking one answer. [Always start by drawing the conjugate base.]
(b)
Problem 52d
Identify the most acidic proton in each pair. Tell which structural features you analyzed and why you weighted them as you did in picking one answer. [Always start by drawing the conjugate base.]
(d)
Problem 52h
Identify the most acidic proton in each pair. Tell which structural features you analyzed and why you weighted them as you did in picking one answer. [Always start by drawing the conjugate base.]
(h)
Problem 53
The nitrogen screened in purple in Figure 4.43 is not protonated at physiological pH. Why?
<IMAGE>
Problem 54c
Identify the Lewis acid and the Lewis base in each of the following reactions.
(c)
Problem 55a
Identify the nucleophile and the electrophile in each of the following reactions.
(a)
Problem 55b
Identify the nucleophile and the electrophile in each of the following reactions.
(b)
Problem 56a
Show an arrow-pushing mechanism that forms the product on the right from the reactant at left. Only one arrow is necessary in each reaction. [Don't forget to draw in the lone pairs on this and the next two assessments.]
(a)
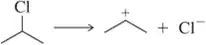
Problem 56d
Show an arrow-pushing mechanism that forms the product on the right from the reactant at left. Only one arrow is necessary in each reaction. [Don't forget to draw in the lone pairs on this and the next two assessments.]
(d)
Problem 57a
Show an arrow-pushing mechanism that forms the product on the right from the reactant at left. Two arrows are necessary in each reaction.
(a)
Problem 57b
Show an arrow-pushing mechanism that forms the product on the right from the reactant at left. Two arrows are necessary in each reaction.
(b)
Problem 58a
Show an arrow-pushing mechanism that forms the product on the right from the reactant at left. Here, three arrows are necessary in each reaction.
(a)
Problem 59c
Identify the arrow types that are shown in each of these arrow-pushing mechanisms.
(iii)
Problem 59d
Identify the arrow types that are shown in each of these arrow-pushing mechanisms.
(iv)
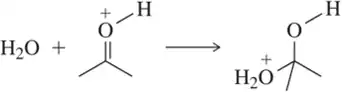
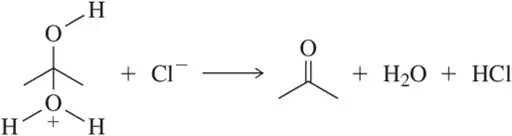
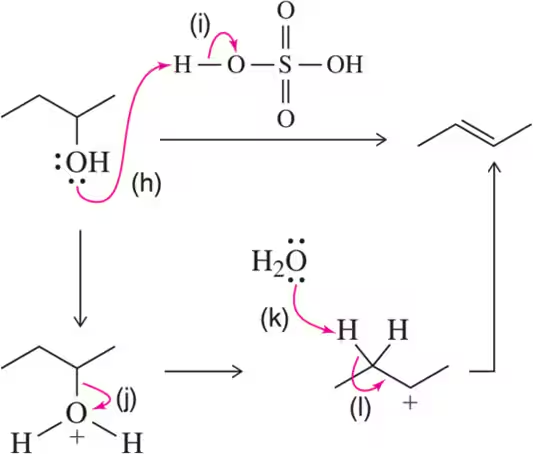
Problem 60a
Identify the arrows shown by type and predict the product that should result.
(a)
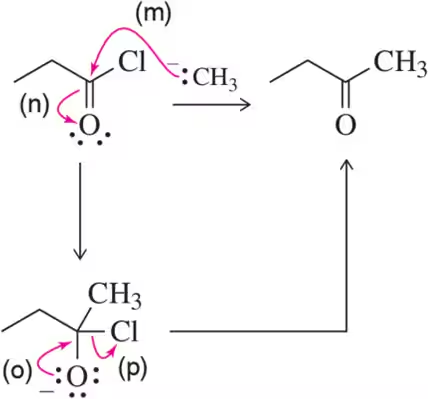
Problem 60e
Identify the arrows shown by type and predict the product that should result.
(e)
Problem 61c
Would you expect the following species to be electrophiles or nucleophiles? Some may be both. Explain your answer.
(c)
Problem 61e
Would you expect the following species to be electrophiles or nucleophiles? Some may be both. Explain your answer.
(e)
Problem 61h
Would you expect the following species to be electrophiles or nucleophiles? Some may be both. Explain your answer.
(h)
Problem 62b(i,ii)
For the following acid–base pairs,
(i) complete the reaction;
(ii) identify the acid (A), base (B), conjugate acid (CA), and conjugate base (CB);
(b)
Problem 62c(i,ii)
For the following acid–base pairs,
(i) complete the reaction;
(ii) identify the acid (A), base (B), conjugate acid (CA), and conjugate base (CB);
(c)
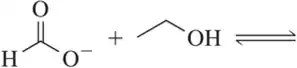
Problem 62e(i,ii)
For the following acid–base pairs,
(i) complete the reaction;
(ii) identify the acid (A), base (B), conjugate acid (CA), and conjugate base (CB);
(e)
Problem 62e(iii)
For the following acid–base pairs, (iii) predict the favored side of equilibrium;
(e)
Problem 62e(iv)
For the following acid–base pairs, (iv) calculate Keq;
(e)
Problem 62e(vi)
For the following acid–base pairs, (vi) draw a reaction coordinate diagram.
(e)