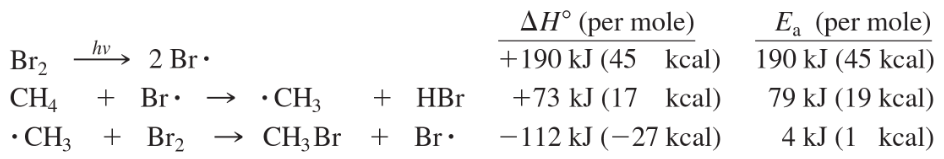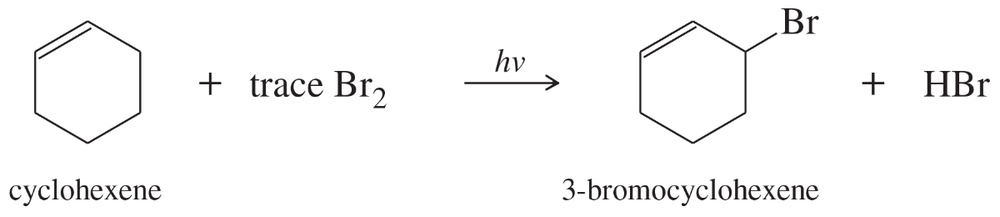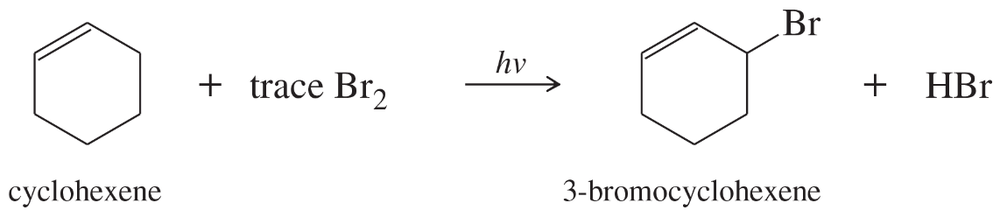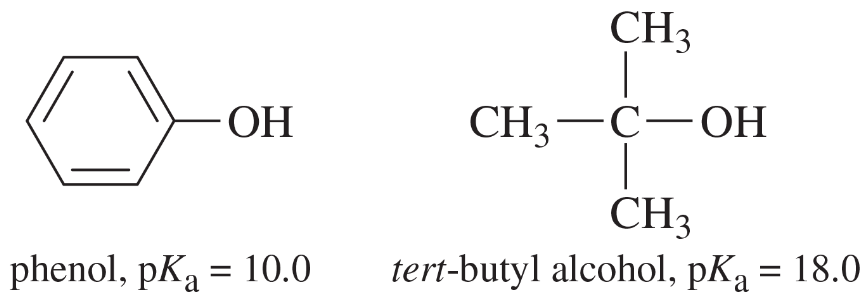Back
BackProblem 16d
The bromination of methane proceeds through the following steps:
d. Compute the overall value of ΔH° for the bromination
Problem 17a,b
a. Using the BDEs in Table 4-2 (page 167), compute the value of ΔH° for each step in the iodination of methane.
b. Compute the overall value of ΔH° for iodination.
Problem 17c
Using the BDEs in Table 4-2 (page 167),
(c) Suggest two reasons why iodine does not react well with methane.
Problem 18
What would be the product ratio in the chlorination of propane if all the hydrogens were abstracted at equal rates?
Problem 19a,b
Classify each hydrogen atom in the following compounds as primary (1°), secondary (2°), or tertiary (3°).
a. butane
b. isobutane
Problem 19c,d
Classify each hydrogen atom in the following compounds as primary (1°), secondary (2°), or tertiary (3°).
c. 2-methylbutane
d. cyclohexane
Problem 19e
Classify each hydrogen atom in the following compounds as primary (1°), secondary (2°), or tertiary (3°).
e. norbornane (bicyclo[2.2.1]heptane)
Problem 22b
Alkoxy radicals (R—O•) are generally more stable than alkyl (R•) radicals. Write an equation showing an alkyl free radical (from burning gasoline) abstracting a hydrogen atom from tert-butyl alcohol, (CH3)3COH. Explain why tert-butyl alcohol works as an antiknock additive for gasoline
Problem 22c
Use the information in Table 4-2 to explain why toluene (PhCH3) has a very high octane rating of 111. Write an equation to show how toluene reacts with an alkyl free radical to give a relatively stable radical.
Problem 25a
In the presence of a small amount of bromine, cyclohexene undergoes the following light-promoted reaction:
a. Propose a mechanism for this reaction.
Problem 25b,c
In the presence of a small amount of bromine, cyclohexene undergoes the following light-promoted reaction:
b. Draw the structure of the rate-limiting transition state.
c. Use Hammond's postulate to predict which intermediate most closely resembles this transition state.
Problem 27
Write an equation for the reaction of vitamin E with an oxidizing radical (RO•) to give ROH and a less reactive free radical.
Problem 28
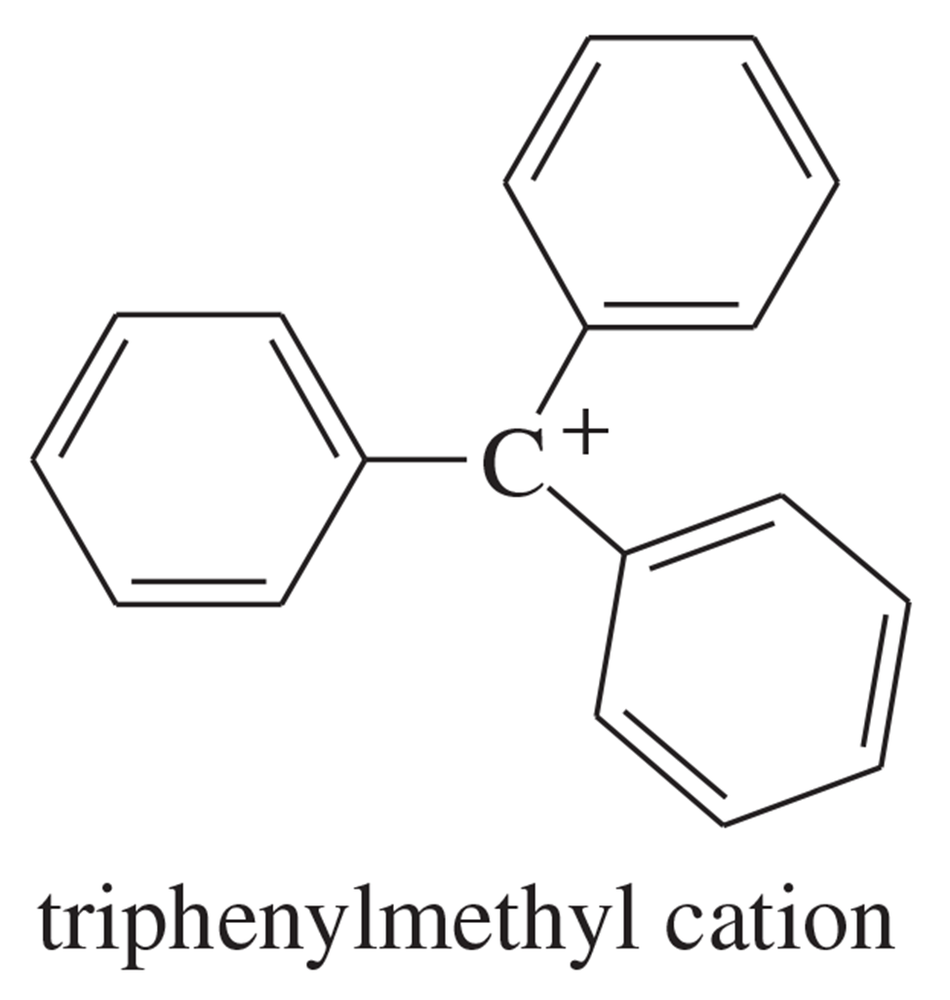
The triphenylmethyl cation is so stable that some of its salts can be stored for months. Explain why this cation is so stable.
Problem 30
Rank the following radicals in decreasing order of stability. Classify each as primary, secondary, or tertiary.
a. The isopentyl radical,
b. The 3-methyl-2-butyl radical,
c. The 2-methyl-2-butyl radical,
d.
Problem 31
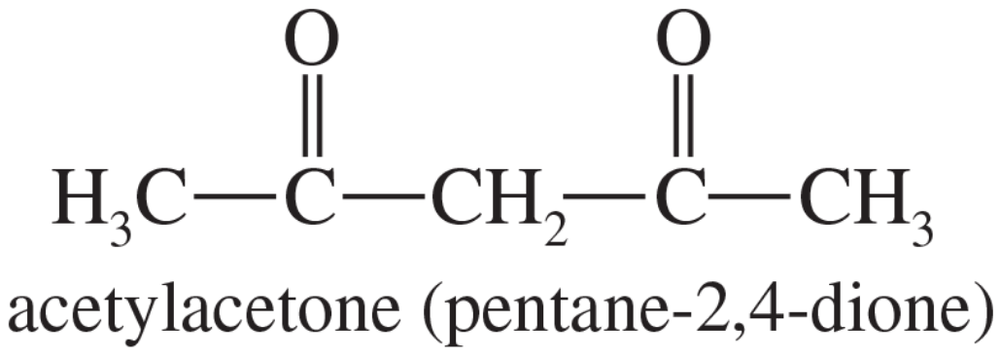
Acetylacetone (pentane-2,4-dione) reacts with sodium hydroxide to give water and the sodium salt of a carbanion. Write a complete structural formula for the carbanion, and use resonance forms to show the stabilization of the carbanion.
Problem 32
Acetonitrile (CH3C≡N) is deprotonated by very strong bases. Write resonance forms to show the stabilization of the carbanion that results.
Problem 33
When it is strongly heated, ethyl diazoacetate decomposes to give nitrogen gas and a carbene. Draw a Lewis structure of the carbene.
Problem 34a
The following reaction is a common synthesis used in the organic chemistry laboratory course.
When we double the concentration of methoxide ion (CH3O–), we find that the reaction rate doubles. When we triple the concentration of 1-bromobutane, we find that the reaction rate triples.
a. What is the order of this reaction with respect to 1-bromobutane? What is the order with respect to methoxide ion? Write the rate equation for this reaction. What is the overall order?
Problem 35
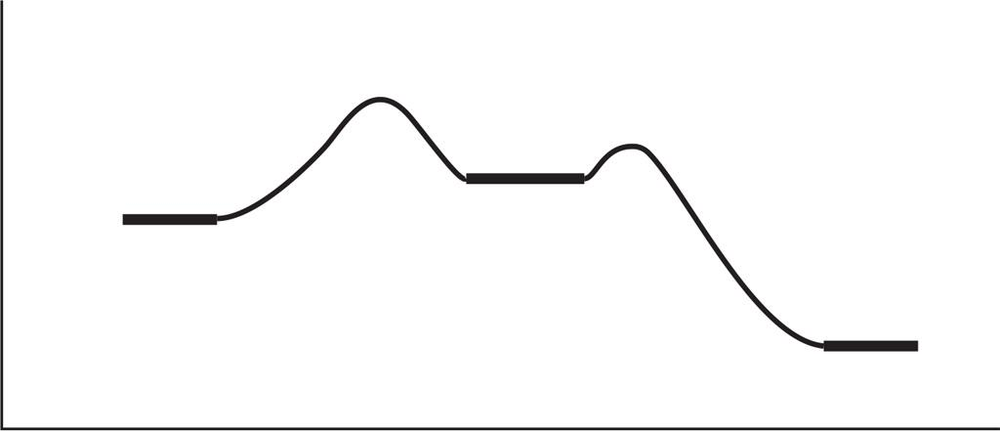
Consider the following reaction-energy diagram.
a. Label the reactants and the products. Label the activation energy for the first step and the second step.
b. Is the overall reaction endothermic or exothermic? What is the sign of ΔH°?
c. Which points in the curve correspond to intermediates? Which correspond to transition states?
d. Label the transition state of the rate-limiting step. Does its structure resemble the reactants, the products, or an intermediate?
Problem 36
Draw a reaction-energy diagram for a one-step exothermic reaction. Label the parts that represent the reactants, products, transition state, activation energy, and heat of reaction.
Problem 37
Draw a reaction-energy diagram for a two-step endothermic reaction with a rate-limiting second step.
Problem 38
a. Draw an approximate reaction-energy diagram for the acid–base reaction of phenol (see below) with 1-molar aqueous sodium hydroxide solution.
b. On the same diagram, draw an approximate reaction-energy diagram for the acid–base reaction of tert-butyl alcohol (see below) with 1-molar aqueous sodium hydroxide solution.
Problem 39
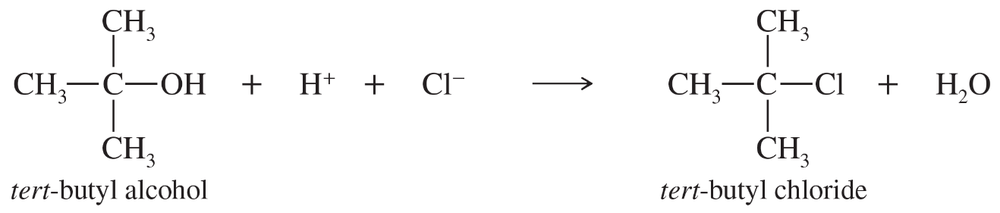
Treatment of tert-butyl alcohol with concentrated HCl gives tert-butyl chloride.
When the concentration of H+ is doubled, the reaction rate doubles. When the concentration of tert-butyl alcohol is tripled, the reaction rate triples. When the chloride ion concentration is quadrupled, however, the reaction rate is unchanged. Write the rate equation for this reaction.
Problem 40a,b
Label each hydrogen atom in the following compounds as primary (1°), secondary (2°), or tertiary (3°).
(a) CH3CH2CH(CH3)2
(b) (CH3)3CCH2CH3
Problem 40c,d
Label each hydrogen atom in the following compounds as primary (1°), secondary (2°), or tertiary (3°).
(c) (CH3)2CHCH(CH3)CH2CH3
(d)
Problem 40e,f
Label each hydrogen atom in the following compounds as primary (1°), secondary (2°), or tertiary (3°).
(e)
(f)
Problem 41a
Use bond-dissociation enthalpies (Table 4-2, p. 167) to calculate values of ΔH° for the following reactions.
a. CH3—CH3 + I2 → CH3CH2I + HI
Problem 41b
Use bond-dissociation enthalpies (Table 4-2, p. 167) to calculate values of ΔH° for the following reactions.
b. CH3CH2Cl + HI → CH3CH2I + HCl
Problem 41c
Use bond-dissociation enthalpies (Table 4-2, p. 167) to calculate values of ΔH° for the following reactions.
c. (CH3)3C—OH + HCl → (CH3)3C—Cl + H2O
Problem 41d
Use bond-dissociation enthalpies (Table 4-2, p. 167) to calculate values of ΔH° for the following reactions.
d. CH3CH2CH3 + H2 → CH3CH3 + CH4